An Unfeasible Challenge or a Profitable Opportunity?
In electrolysis projects, hydrogen is the main focus, as it is the most valuable product and is considered key to decarbonization. However, this process also generates large volumes of oxygen as a byproduct. In terms of weight, for every kilogram of hydrogen produced, 8 kg of oxygen are generated.
Often, this oxygen is not utilized and is safely released into the atmosphere. Nevertheless, its valorization can improve the project’s economics and optimize industrial processes such as combustion with carbon capture.

llustration 1: PEM Electrolyzers (Air Liquide, 2024).
This article will explain how oxygen valorization can be integrated into hydrogen projects and what aspects must be considered to ensure that such valorization is viable, since it is not always possible to make this element profitable.
How Is Oxygen Currently Produced and Marketed?
Oxygen is a colorless, odorless, and tasteless gas that, due to its low density, is generally stored and transported in liquid form at temperatures below -183 °C. Its high reactivity makes it an essential oxidizing agent in multiple industries. Although it is not flammable, it is a strong oxidizer that reacts with almost all organic materials and metals, typically forming an oxide. This makes the selection of suitable materials crucial when handling this element.
Table 1: Properties of Oxygen (Air Products, 2024).
| Parameter | Value | Units |
| Molecular weight | 32 | g/mol |
| Boiling point | -183 | ºC |
| Freezing point | -218,8 | ºC |
| Critical temperature | -118,4 | ºC |
| Critical pressure | 49,6 | bar |
| Density, liquid (1 bar) | 1.141 | kg/m3 |
| Density, gas (20 ºC and 1 bar) | 1,33 | kg/m3 |
| Latent heat of vaporization | 213 | kJ/kg |
Regarding its current production through conventional technologies, cryogenic air distillation is the most widely used method for producing oxygen in large volumes, allowing for high purity levels. For lower flow rates, pressure swing adsorption (PSA/VPSA) is used, although it results in lower purity.
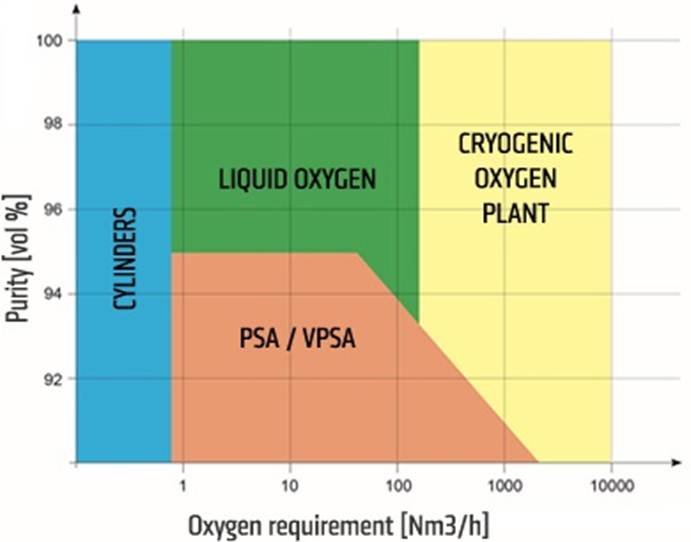
Illustration 2: Methods of Oxygen Production and Supply Based on Required Gas Purity and Flow Rate (Omega Air, 2024).
Based on the differences in investment and operational costs associated with each technology, the cost of oxygen production will vary significantly depending on the production method, as shown in Illustration 3.
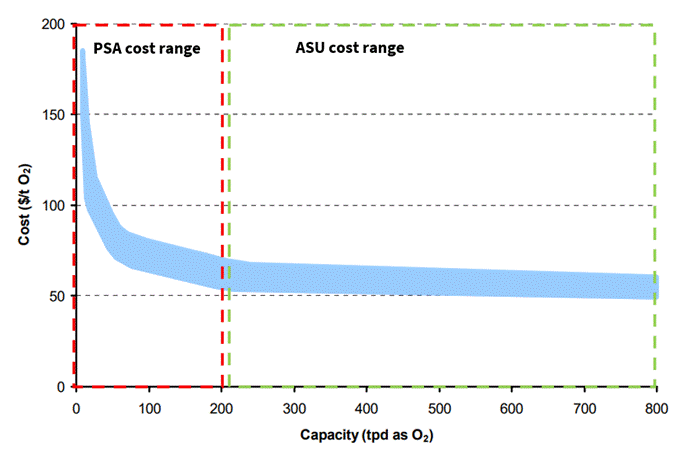
Illustration 3: Cost Comparison of Oxygen Produced by PSA and Cryogenic Distillation (Doug Palfreyman, Aaron Cottrell, Peter Scaife & Louis Wibberley, 2006).
Currently, oxygen is used across a wide range of sectors, from the medical field to its role as an oxidant in high-temperature furnaces, in the pulp production process, or in wastewater treatment:
- Medical sector: Medical oxygen for patients with respiratory insufficiency.
- Metallurgical industry: Used in cutting and welding processes.
- Water treatment: Oxygenation for the removal of contaminants.
- Industrial combustion: Enhances the efficiency of furnaces and boilers.
- Pulp and paper industry: For delignification and bleaching of pulp.
- Chemical industry: Oxygen reacts with hydrocarbons to form aldehydes and alcohols.
Oxygen Production in Water Electrolysis: How It Works and What Factors Influence Its Valorization
With the rise of electrolysis for renewable hydrogen production, some facilities could be located near potential oxygen consumers, opening the door to commercializing this byproduct. In an ideal scenario, this strategy would help reduce hydrogen costs and generate industrial synergies.
One example of application is the use of oxygen to enrich combustion air in biomass boilers, which facilitates CO₂ capture by increasing the concentration of the molecule in the flue gases. Later, this CO₂—captured at a lower cost thanks to the use of oxygen—can be combined with renewable hydrogen to produce synthetic fuels at a lower cost than similar processes that do not involve oxygen.
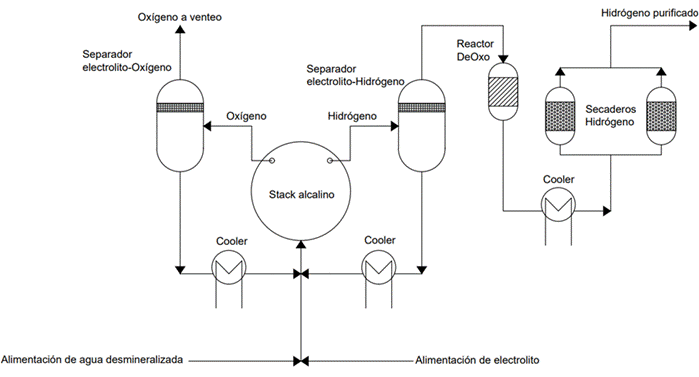
Illustration 4: Process Diagram of Hydrogen and Oxygen Production via Water Electrolysis.
The potential for Oxygen valorization in renewable hydrogen projects, in most cases, the oxygen generated as a byproduct is vented, with a cost assumption of €0/kg O₂. This occurs because the profitability of the plant is based exclusively on hydrogen sales, whose price is calculated through the Levelized Cost of Hydrogen (LCOH).
However, commercializing oxygen represents an opportunity for additional revenue, improving the economic viability of water electrolysis. Therefore, by integrating oxygen valorization, it becomes possible to optimize the profitability of water electrolysis plants.
Table 2: Hydrogen and Oxygen Production Capacities Based on Electrolyzer Power
| Electrolyzer Power (MW) | Oxygen Production (t/year) | Hydrogen Production (t/year) |
| 1 | 728 | 91 |
| 50 | 36.364 | 4.545 |
| 100 | 72.728 | 9.091 |
| 200 | 145.455 | 18.182 |
| 300 | 218.182 | 27.273 |
The valorization potential of oxygen generated by electrolysis is determined by system characteristics, including installed power, oxygen outlet pressure, and the plant’s morphology and design. These factors influence both the quantity and purity of the oxygen produced, key aspects when assessing potential demand and processing costs.
The lower the oxygen purity and the more demanding the end-use application, the higher the treatment costs. In some cases, additional processes such as drying or even hydrogen removal may be required, especially if significant hydrogen permeation occurs.
Oxygen Pressure and Purity in Electrolysis: Key Factors for Its Utilization
The pressure and temperature conditions of the oxygen produced by electrolysis vary depending on the technology used. In general, pressure can range from 0 to 30 barg, while temperature may reach up to 70 °C.
In the case of alkaline electrolysis, oxygen is typically generated at higher pressures than in PEM electrolysis systems. This is because PEM electrolyzers, which use a proton exchange membrane, allow for differential pressure operation, meaning the oxygen side generally operates near atmospheric pressure. However, some PEM technology manufacturers have developed systems capable of reaching pressures close to 10 barg.
On the other hand, alkaline electrolyzers can operate with oxygen pressures up to 30 barg, making them a more suitable option for applications that require compressed oxygen without the need for additional pressurization systems.
Illustration 5: SIAD Oxygen Compressor (SIAD, 2025).
The oxygen produced by electrolysis may contain impurities, primarily water (as it exits the electrolyzer saturated) and traces of hydrogen. The latter poses a critical safety concern, as excessive hydrogen crossover into the oxygen stream can create a flammable mixture. To mitigate this risk, it is essential to use high-quality equipment and technologies that minimize hydrogen permeability in the system.
As for the water present in the oxygen, it usually does not pose a problem in applications such as combustion. However, if the oxygen needs to be liquefied, it is crucial to dry it beforehand to avoid ice formation in the equipment. Additionally, in such cases, the hydrogen concentration must be reduced to levels of 500–1000 ppm to ensure a safe and efficient process.
Operating the electrolyzer under pressure on the oxygen side offers significant advantages in terms of efficiency and operating costs. First, it substantially reduces the water content in the gas, which facilitates drying and minimizes the impact of moisture on downstream processes.
Furthermore, this strategy optimizes energy consumption in subsequent liquefaction or compression stages, lowering the energy demand for transporting liquefied oxygen or using it in compressed form. This translates into lower operating costs and greater competitiveness of electrolytic oxygen compared to conventional production methods. As shown in Table 3, operating under pressure helps minimize the costs associated with drying, improving process efficiency by reducing moisture content.
In addition to water and hydrogen, if alkaline technology is used, it is essential to monitor the presence of KOH in the oxygen line, as it can cause corrosion. Typically, compressors require the water concentration to be below 10 ppmv and the maximum KOH content not to exceed 5 mg/Nm³ to prevent equipment damage.
Table 3: Water Composition in the Oxygen Stream as a Function of Pressure
| O₂ Pressure (bara) | Water Composition (%mol) | O₂ Composition (%mol) |
| 1 | 31,1 | 68,9 |
| 10 | 3,1 | 96,9 |
| 15 | 2,1 | 97,9 |
| 20 | 1,6 | 98,4 |
| 25 | 1,2 | 98,8 |
| 30 | 1,0 | 99,0 |
When oxygen requires additional treatment, such as liquefaction for transport or compression to meet usage conditions, the production capacity becomes a key factor in the final cost of oxygen.
In small-scale electrolysis systems (<5 MW), valorization costs rise significantly, as investment in treatment equipment (CAPEX) cannot be efficiently amortized over the plant’s lifetime due to low production volumes.
This impact is even more significant in liquefaction and compression processes, where oxygen compression represents a considerable economic penalty. This is due to the high cost of compression systems and their elevated energy consumption, which can reach 500 kWh per ton of O₂ to obtain it in liquid phase.
How Do the Morphology and Design of Electrolysis Plants Affect Oxygen Valorization?
Oxygen valorization in electrolysis systems largely depends on the capacity of the facility. In small-scale projects, which typically use containerized solutions, the equipment design generally does not account for the commercialization of oxygen, which may render its utilization technically and economically unfeasible. In such cases, specific studies are required to ensure that oxygen valorization does not compromise the safe operation of the electrolyzer.
This issue is particularly critical when oxygen is generated at atmospheric pressure, as there is a higher risk of backpressure that could compromise the equipment’s safety. Therefore, before considering oxygen valorization, it is essential to verify that electrolyzer manufacturers support this option and that there are no technical or economic limitations related to the oxygen generation pressure.
Main Challenges for Commercializing Oxygen in Electrolysis
Even though the oxygen production cost may be considered negligible in hydrogen projects, there are certain challenges that must be addressed to ensure that oxygen valorization is feasible, primarily:
- Offtaker.
- Required Treatment.
Oxygen Offtaker Assessment: Keys to Commercialization
The commercialization of oxygen requires strategic planning, as many nearby consumers are bound by long-term supply contracts, making it difficult to establish new agreements. Additionally, the oxygen market is highly saturated and dominated by large industrial gas companies, which limits the entry of new players.
For this reason, it is crucial to evaluate oxygen valorization opportunities and determine whether a viable business model truly exists. In this context, oxygen valorization becomes especially attractive when there is potential for integration with new Power-to-X projects or with oxygen-consuming industries that are not tied to rigid contracts or that can terminate agreements without penalties for the offtaker.
Treatment of Oxygen Produced via Electrolysis: Impact on Costs and Economic Competitiveness
Although the oxygen generated through electrolysis can be considered cost-free, its final price will depend on the treatment required for its use. In this regard, two key factors determine its commercial viability: the required purity and the scale of demand.
Water electrolysis is the best option for applications that require high-purity oxygen and large-scale production. Renewable hydrogen projects, operating in ranges of tens to hundreds of megawatts (MW), can generate thousands of tons of oxygen per year, making it attractive for industrial sectors with high quality requirements.
On the other hand, for lower production capacities and moderate purity requirements, PSA technologies offer a more cost-effective and efficient alternative, adapting to smaller-scale demand with lower operating costs.
To illustrate the impact of electrolyzer size on oxygen valorization, the levelized cost of oxygen can be calculated based on plant size (as shown in Table 4) and the treatment process required for offtake either liquefaction or compression.
In this context, it is essential to consider that, in oxygen valorization from water electrolysis, two fundamental factors determine the business viability: the distance to the consumer and the treatment required for final use.
If the distance is considerably large, transportation costs rise, favoring on-site production through conventional technologies such as PSA/VSA systems. Conversely, in the ideal scenario, the consumer is located on the same site or within a few kilometers of the oxygen source. In such cases, a moderate compression system (10–15 bar) allows for oxygen valorization without significant cost impact, making it competitive compared to traditional methods.
When the distance exceeds several tens or hundreds of kilometers, liquefaction becomes the most viable alternative for oxygen transport. However, this process entails a greater cost penalty, due to both the high energy consumption and the initial investment in liquefaction infrastructure and cryogenic storage.
Table 4: Oxygen Production Capacity Based on Different Electrolysis Power Levels, Assuming 55 MWh/t H₂ Efficiency and 5,000 Operating Hours per Year
| Electrolysis Capacity (MW) | O2 Production (t/year) | H2 Production (t/year) |
| 1 | 727,27 | 90,91 |
| 50 | 36.363,64 | 4.545,45 |
| 100 | 72.727,27 | 9.090,91 |
| 200 | 145.454,55 | 18.181,82 |
| 300 | 218.181,82 | 27.272,73 |
Based on the estimated production values and a relatively simple economic model, starting from estimated investment costs of the equipment and their energy penalties to calculate operating costs, the cost associated with each treatment process can be estimated.
In the case of liquefaction, assuming an electricity cost of €40/MWh, the levelized cost of liquefied oxygen ranges from €100/t O₂ at 5 MW capacity to €40/t O₂ at 200 MW.
On the other hand, for compression, also assuming an electricity cost of €40/MWh, the levelized cost to compress oxygen to a moderate pressure of 15 bar ranges from €55/t O₂ (5 MW) to €15/t O₂ (200 MW). For high-pressure compression (e.g., 200 bar for transport in gas phase), the cost increases to €75/t O₂ (5 MW) and €25/t O₂ (200 MW).
Conclusion
Hydrogen production through water electrolysis generates oxygen as a byproduct in large quantities. Its valorization can become an additional source of income and improve the efficiency of industrial processes, such as CO₂ capture.
However, the viability of this strategy depends on several key factors, such as the presence of nearby consumers and the conditions of the oxygen at the electrolyzer outlet. These aspects determine both the existence of a viable market and the competitiveness of the oxygen supply from electrolysis compared to conventional methods such as PSA systems.
In this way, despite its potential, oxygen valorization faces certain challenges:
- Purification and compression costs: If the O₂ produced requires the removal of oxygen, water, and/or compounds such as KOH (in alkaline electrolyzers) in a concentration higher than recommended, the cost of valorization increases. Therefore, the necessary intensity of treatment will determine its final cost and, therefore, the competitiveness of its supply through electrolysis versus conventional methods.
- Offtake: Not all potential nearby consumers will be able to accept the oxygen, even at a lower price than they currently pay, as they may be bound by long-term contracts. Therefore, a proper feasibility study for the sale of oxygen is essential, and to ensure that a market for the oxygen produced in electrolysis truly exists.
- Distribution logistics: The best case for valorization will be when the oxygen is consumed in the same place where it is produced or nearby, as could be a capture plant linked to an electrolysis project for fuel production. This is because in that case, with simple compression (up to a moderate pressure), oxygen valorization could be carried out, reducing costs and obtaining a low oxygen cost, capable of competing with and outperforming conventional production processes even at low electrolysis power levels. On the other hand, transport in liquid state implies significant costs, so when liquefaction is necessary for transport, the competitiveness of oxygen decreases and, to be competitive with conventional supply, the installed electrolysis capacity must be higher.
o achieve successful development, it is essential to analyze these factors, as they allow determining the viability of oxygen valorization and maximizing the profitability of the project. In addition, a strategic evaluation ensures that there is a real demand and a market share for the oxygen generated as a byproduct of electrolysis.
💡 Interested in optimizing your renewable hydrogen project? Contact us to receive advice on oxygen valorization and improve the profitability of your electrolysis plant.
🔗 More about renewable hydrogen at: AtlantHy Academy
References
Air Liquide. (2024). PEM electrolyzers to produce renewable hydrogen: how does it work? Obtenido de: https://www.airliquide.com/stories/hydrogen/pem-electrolyzers-produce-renewable-hydrogen-how-does-it-work
Air Products. (2024). Liquid Oxygen. Obtenido de: https://www.airproducts.com/gases/liquid-oxygen
Doug Palfreyman, Aaron Cottrell, Peter Scaife & Louis Wibberley. (2006). TECHNO-ECONOMICS OF OXYGEN-FIRED PF POWER GENERATION WITH CO2 CAPTURE. Retrieved from https://www.researchgate.net/publication/238079799_TECHNO-ECONOMICS_OF_OXYGEN-FIRED_PF_POWER_GENERATION_WITH_CO2_CAPTURE?enrichId=rgreq-5c8be06cf66f7508efbb35f1d84c9caf-XXX&enrichSource=Y292ZXJQYWdlOzIzODA3OTc5OTtBUzoxMzQyMTIwMzIxNDMzNjBAMTQwOTAxMDA1MDQ2M
Omega Air. (2024). Nitrogen and oxygen production. Obtenido de: https://www.omega-air.si/news/news/nitrogen-and-oxygen-production
SIAD. (2025). Oxygen Compressors. Obtenido de: https://www.siadmi.com/oxygen-compressor