Tackling climate change requires more than just reducing carbon dioxide (CO2) emissions; we need innovative solutions that align with our lifestyle without compromising it. In this context, carbon capture emerges as one of the most promising technologies, offering an effective strategy to remove CO2 directly from the atmosphere or capture it before it even reaches it.
How? In this article from AtlantHy Academy, we explain it to you.
Introduction
For years, the focus has been on eliminating carbon dioxide from our lives, businesses, economic activities, and society in general. This is because it carries negative connotations, being the main driver of climate change due to the more than 37 billion tonnes that humans generate globally, which end up in the atmosphere.
Due to the adverse effects that climate change has on society, carbon neutrality must be achieved by 2050. This temporal horizon is set because it is the scenario that would allow limiting the temperature rise to 1.5°C above pre-industrial levels, thus avoiding the terrible consequences of climate change, as described in 2018 by the IPCC (Intergovernmental Panel on Climate Change).
That said, you might think: Why aim for carbon neutrality and not complete elimination of CO2 emissions? This question has an easy answer: although carbon dioxide is conceived as a problem, it can also be fundamental in achieving climate goals. Since it contains carbon in its structure, it opens up a range of possibilities in the so-called hard-to-abate sectors. These are sectors where decarbonization through direct electrification with renewable energy is not technically or economically viable. Therefore, it is necessary to opt for renewable fuels and raw materials, which will largely be produced from the combination of hydrogen with carbon dioxide.
This approach can yield fuels like synthetic kerosene or methanol to decarbonize aviation and maritime transport, as well as produce various chemical products (e.g., methanol to olefins, methanol to formaldehyde) in the industry. This not only helps decarbonize transport and chemical production but also generates significant revenue for CO2 emitters.
Considering the scale of hard-to-abate industries and their energy and raw material demands, achieving carbon neutrality will require substantial amounts of CO2, both to eliminate intrinsic emissions and to produce recycled or renewable fuels that reduce the carbon intensity of transport and industry.
Fortunately, carbon capture, utilization, and storage (CCUS) technologies which involve capturing, storing, and fixing CO2 have been in use for a long time. Thus, they can be rapidly implemented to help industries meet their objectives.
This capture can be carried out not only at emission sources, such as power generation or industrial facilities that use fossil fuels or biomass, but also to capture CO₂ present in the atmosphere. In the latter case, since the concentration of CO₂ is much lower than in cases where the process is carried out at emission sources, costs increase, relegating the DAC (Direct Air Capture) technology to pilot projects for the time being.
The hopes placed on these technologies for carbon elimination and capture are enormous, with expectations that up to 10% of emission reductions from 2021 to 2050 will come from the application of CCUS technologies. Once captured, CO₂ could be used as a raw material in countless applications, as shown in the following diagram (Mertens, 2023).
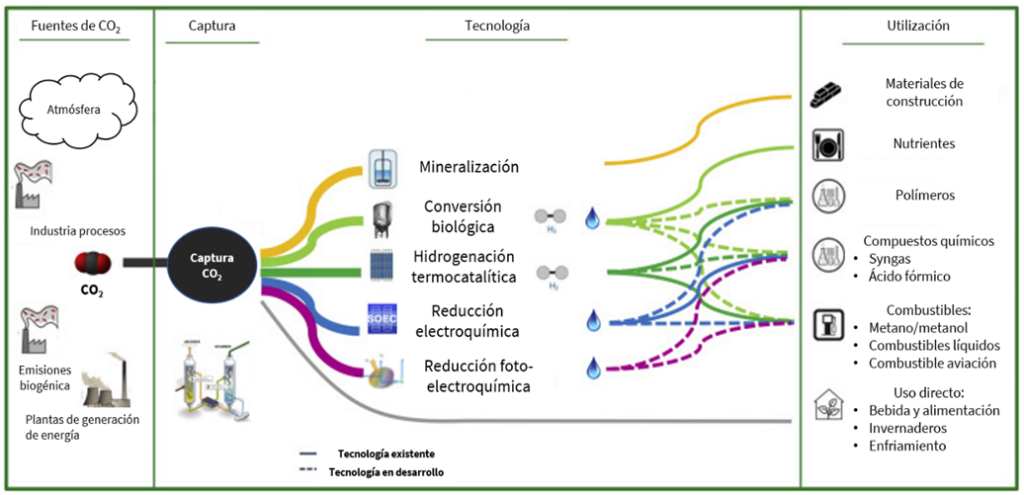
Illustration 1 Carbon dioxide value chain. Adapted from: (Mertens, 2023).
Currently, there are approximately 45 commercial facilities applying CCUS to industrial processes, fuel transformation, and power generation, with a total annual capture capacity of nearly 50 Mt of CO2. Additionally, there are around 700 projects in various stages of development across the CCUS value chain, aiming to capture more than 435 Mt of CO2 per year.
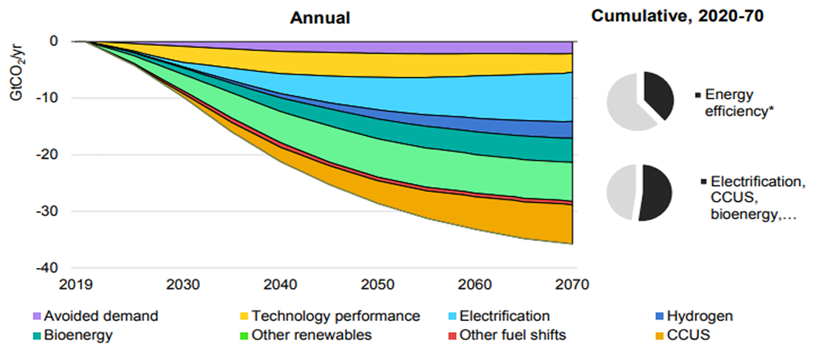
Illustration 2 Expected decline in emissions in the coming decades and responsible technology (International Energy Agency, 2023).
Most industrial carbon dioxide emission sources are sources that generate large flows of exhaust gases in which the CO2 concentration is low due to the presence mainly of nitrogen from the air necessary for combustion (Table 1).
Table 1 Typical CO2 characteristics in exhaust gases from different units and processes (Wang, 2020).
| Exhaust Gas Source | CO₂ Concentration (%vol) |
| Gas turbine | 3 – 4 |
| Oil refinery boiler | 8 |
| Gas boiler | 7 – 10 |
| Oil boiler | 11 – 13 |
| Coal boiler | 12 – 14 |
| IGCC | 12 – 14 |
| Hydrogen production | 15 – 20 |
| Steel production | 20 – 27 |
| Aluminium production | 1 – 2 |
| Cement production | 14 – 33 |
Since both the energy demand (which translates into OPEX) and the dimensions and, therefore, the investment needs (CAPEX) of CO2 capture equipment increase with the decrease in CO2 concentration, it is preferable to carry out the capture in highly concentrated streams to simplify the process and reduce costs. The higher the concentration, the easier the capture process and the more competitive the costs. The following image from the IEA relates the concentration of various sources for CO2 capture with the capture cost in them, clearly showing how costs become much more competitive for processes in which the carbon dioxide concentration is high.
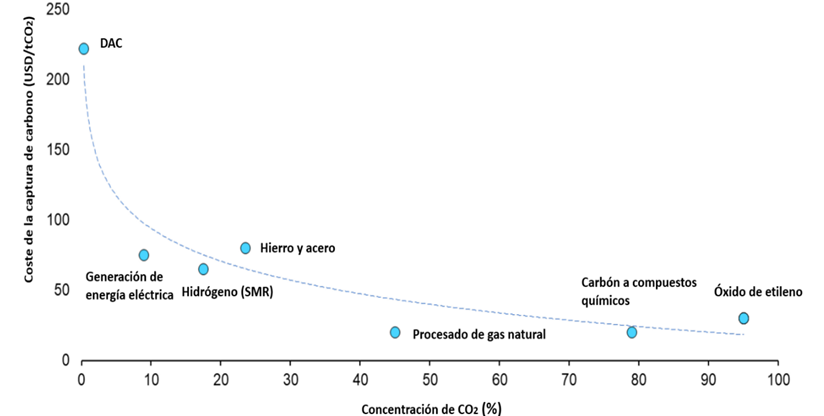
Illustration 3 Estimated CO2 capture cost values for different carbon dioxide concentrations in exhaust gas. Adapted from (IEA, 2022).
urrently, several technological pathways are followed for CO2 separation and capture. These technologies differ from each other depending on the location of the carbon capture stage, as it can be carried out before or after the combustion process, and depending on the oxidant used.

Illustration 4 AVR carbon capture plant in Duiven (AVR, 2024).
Thus, the main types of carbon capture are:
Post-combustion: CO2 separation from gases is carried out after the combustion process. Since combustion uses air as an oxidant, these gases have a low partial pressure of CO2 (0.03-0.2 bar) and/or a low CO2 concentration (3-20% vol) (Wang, 2020). Some of the main technologies considered within this category are:
- Capture through chemical absorption: A chemical reaction occurs betweenCO2 and a specific compound, followed by desorption in an opposite process that generally requires energy input, usually in the form of thermal energy. This type of system with chemical solvents has been used in industrial gas streams since the 1930s in natural gas processing. The main technologies in this field are amines or carbonates, with the former being the most used. These processes absorb CO2 at low temperatures and release it at high temperatures, achieving a capture of 80-95% with a purity of 99.95%. The process requires equipment such as absorption and desorption towers.
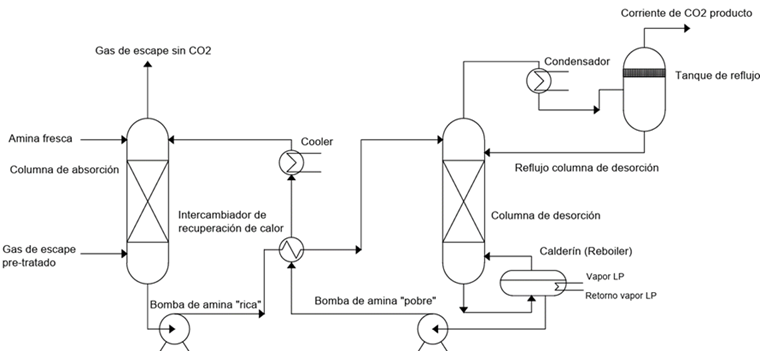
Illustration 5: Process diagram of an amine capture system (Own elaboration).
- Capture via Chemical (TSA) and Physical (PSA and VSA) Adsorption: These processes use physical and chemical forces to capture CO2 molecules from a gas stream, which are then released under high-purity conditions by reversing those forces. These technologies are especially suited for gas streams with high CO2 concentrations (Global CCS Institute, 2021). Today, PSA (Pressure Swing Adsorption) is the most commonly used method to separate CO2 from hydrogen in steam methane reformers.

Illustration 6: PSA units by Linde for H2 and CO2 capture in Germany (Linde, 2024).
- Membranes: Widely used in high-concentration plants, membranes act as barriers that separate different compounds in a gas mixture based on their ability to permeate through the membrane. This separation method uses partial pressure as the driving force and is most effective when the gas mixture is fed at high pressure (Global CCS Institute, 2021).
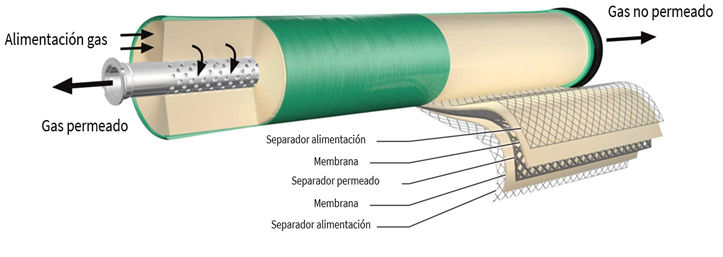
Illustration 7: Diagram of a membrane used for gas capture. Adapted from (SLB, 2023).
- Cryogenics: Cryogenic CO2 capture involves physical separation based on differences in boiling points and desublimation properties of the gas components. It can be paired with a PSA-based pre-concentration stage (Font-Palma, 2021). This method produces highly pure, liquefied CO₂ (suitable for industrial or food-grade use), and unlike amine systems, does not require a separate liquefaction plant for transport, making it ideal for long-distance shipping.
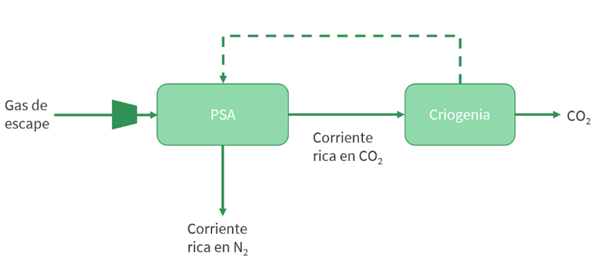
Illustration 8: Diagram of cryogenics + PSA process (own elaboration).
- Chemical Looping: An emerging technology similar to oxy-combustion, chemical looping generates a concentrated CO2 stream. It removes the need for separating CO2 from the fuel (as in pre-combustion) or flue gas (as in post-combustion), and is being extensively studied for application in cement production (Wang, 2020).
- Pre-combustion: This approach is attractive for capturing or utilizing CO2 because it allows for process design from the outset. It captures CO2 before combustion and is typically used in processes that produce syngas (CO + H2) via gasification of fuels (coal, gas, biomass) or natural gas reforming. The syngas then undergoes a water-gas shift (WGS) reaction to convert CO into more H2 and CO2 , increasing CO2 concentration and simplifying separation from hydrogen (Wang, 2020).
- Oxy-combustion: This is a variation of post-combustion capture. It addresses the main challenge of post-combustion—high nitrogen content (about 80–90% of flue gas)—by using pure oxygen instead of air. This reduces flue gas volume, increases CO2 concentration, and lowers capture costs and system size. Although promising, oxy-combustion faces technical challenges and limited commercial availability for industrial boilers and furnaces (Wang, 2020). Oxy-combustion has been attracting increasing interest lately, especially due to the opportunity to make use of oxygen generated during electrolysis oxygen that is typically vented into the atmosphere. However, this approach presents several technical challenges for boiler engineers and industrial furnace manufacturers, which is why there are currently limited commercial solutions available on the market for implementing this type of combustion in industrial facilities.
All of these technologies require significant capital investment, but operational costs are also notable particularly in electricity, and in some cases (e.g., chemical absorption), large thermal energy demands, which can raise long-term costs.
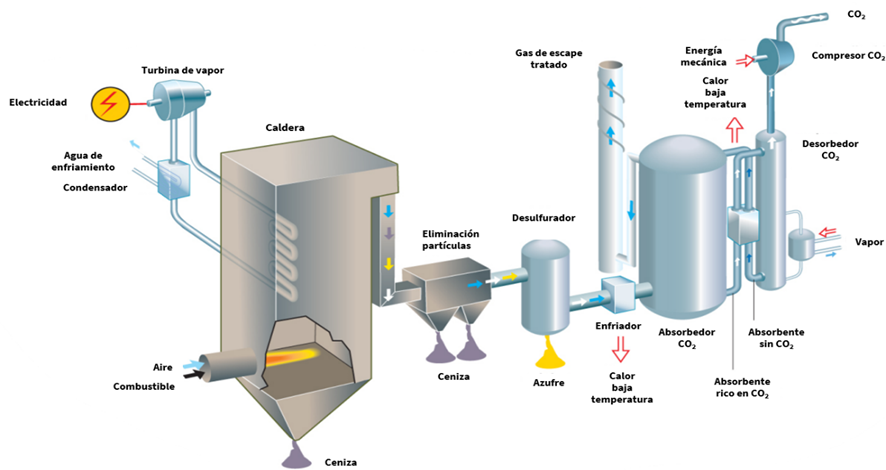
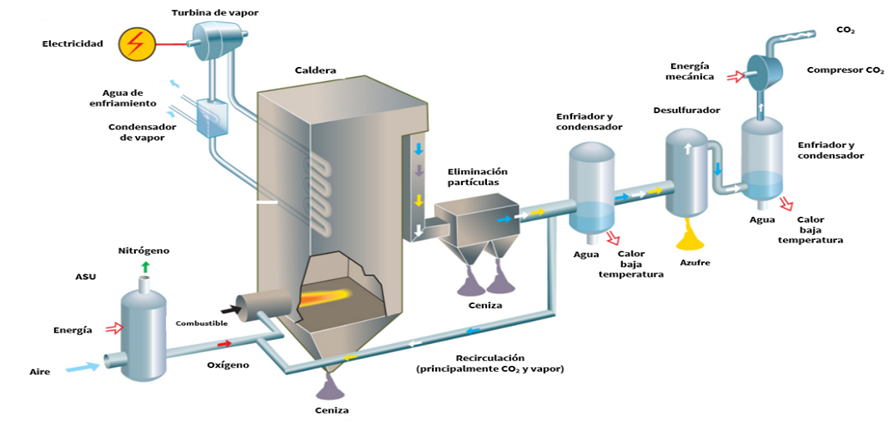
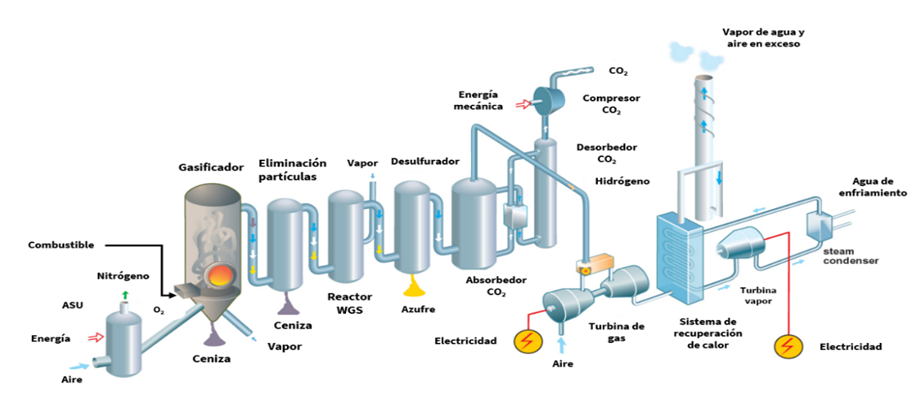
Illustration 9: Post-, oxy-, and pre-combustion technologies (Vattenfall).
As previously mentioned, there are multiple technologies, each with its own advantages and disadvantages. The choice of the most appropriate process depends heavily on the specific conditions of the plant where capture is proposed, as well as other key aspects such as:
- Technological maturity
- Integration with existing facilities
- End-user of the CO₂
- Public funding
Technological Maturity
Whenever there is a shift in any field, there is natural hesitation around using emerging technologies or those proven in other sectors but not yet tested in new applications. One of the main factors influencing the chosen technology is technological risk. In this respect, amines and PSA systems are the most proven at commercial scale. However, industries are increasingly open to trying newer technologies like oxy-combustion or cryogenics, due to their synergies and potential to improve competitiveness in the carbon capture market.
Existing Facilities
Because many capture projects will be implemented in existing plants (e.g., paper mills, cement factories, petrochemical plants, biomass plants), it’s essential to evaluate the compatibility of the capture system with current processes. In many cases, the optimal capture solution might conflict with existing industrial activity. Therefore, a compromise must be found between the ideal capture technology and the solution that can realistically be integrated into the plant. Key considerations include:
- Available space
- Maximum permitted height
- Equipment and processes where CO₂ is generated, which might require major modifications
It’s also critical to explore possible synergies between capture systems and current processes. For instance, post-combustion systems using solvents require thermal energy, which could be supplied by residual heat from the plant—if it’s of sufficient quality. This would reduce operational expenses and improve the economic competitiveness of the captured CO2.

Illustration 10: Heat exchange equipment in a CO2 capture plant in Soria (own image).
End-User of the CO₂
Once CO2 is captured, it must be transported to the end-user under specific conditions and with the required purity. The final use of the CO2 must be considered during the design of the capture plant. The selected technology should guarantee that the CO2 quality meets the requirements of its final use, and if transport is needed, the system must be able to condition the CO2 accordingly—whether liquefied for shipping or trucking, or compressed for pipeline transport.
Thus, a technology with slightly higher capture costs might be preferable if it delivers CO2 in a form more suited for its final use or transport—such as cryogenic capture, which can compete with amines at low concentrations despite higher initial costs. Its key benefit: it provides liquid-phase CO2 directly, avoiding the need for a separate liquefaction plant for international transport.
Another critical factor is the carbon footprint of the captured CO2. For instance, in synthetic fuel production, the origin of CO2 determines whether the fuel remains classified as renewable. A captured CO2 with high carbon intensity could disqualify the final product. Fuels are no longer considered renewable if:
- The CO2 comes from a non-biogenic source: all CO2 is valid until 2035; post-2035, emissions from power generation will be disqualified, and from fossil fuels or industrial processes (like cement) until 2041.
- The final fuel (RFNBO) doesn’t reduce emissions by at least 70% compared to a benchmark of 94 gCO₂/MJ.
Capturing CO₂ doesn’t automatically make it carbon neutral, as electric and thermal energy used in the process must come from renewable sources. Projects will favor CO₂ with a low, zero, or even negative footprint. Thus, knowing the buyer of the molecule is crucial to project design.
Public Funding
Projects of this nature involve substantial capital investments and long-term commitments. Public funding can make it possible to choose a higher-capex solution with lower operational costs. Governments generally don’t restrict funding by technology, so the OPEX becomes a key differentiator with very different energy demands between solvent-based, cryogenic, or oxy-combustion capture systems.
Receiving aid can tilt a project toward higher upfront investment but lower OPEX, which pays off over time as long as the chosen technology is technically viable

Illustration 11: View of an industrial carbon capture plant (own image).
Conclusion
The CCUS industry is not new. Many technologies are already commercially proven and operationally sound. Others are still in development but offer significant potential in future energy and industrial systems. However, these must first prove commercial viability to be scaled up across industry.
While carbon capture might seem less exciting than green hydrogen, the two technologies are closely linked. CO2 is essential for hydrogen to reach its full potential in synthetic fuel and chemical production, which will be key to decarbonizing hard-to-abate sectors. Moreover, capture technologies can remove emissions directly from the air or from industrial processes (e.g., cement), helping meet 2050 neutrality targets.
With ambitious climate goals and strong potential, many CCUS projects will emerge in the near and medium term, establishing networks for CO2 transport, trade, and storage. Understanding this field and anticipating future needs will prepare society to make smart, informed decisions based on solid analysis and good judgment.
🎧 Check out Episode 25 of our podcast: CO2 and Hydrogen with Álvaro Reyes (Orchestra Scientific & Arcamo Group) for more insights into these technologies.
At AtlantHy, we help companies develop carbon capture projects and already have successful experiences with various sizes and technologies. We’re truly passionate about this technology.
👉 Get in touch with us to bring your project to life!
If you enjoyed this article, stay tuned for future posts where we’ll dive deeper into energy consumption, investment costs, integration strategies with RFNBO plants, and more.
Follow us at AtlantHy Academy!
References
AirLiquide. (2023). Cryocap Carbon Capture Technologies.
AVR. (Agosto de 2024). First tons of CO2 captured from residual waste supplied to greenhouse horticulture. Obtenido de https://www.avr.nl/en/first-tons-of-co2-captured-from-residual-waste-supplied-to-greenhouse-horticulture/
Font-Palma, C. C. (2021). Review of Cryogenic Carbon Capture Innovations and Their Potential Applications. Journal of Carbon Research.
Global CCS Institute. (2021). Technology Readiness and Costs of CCS.
IEA. (2022). Direct Air Capture: A key technology for net zero.
International Energy Agency. (2023). Carbon capture, utilisation and storage. Obtenido de https://www.iea.org/fuels-and-technologies/carbon-capture-utilisation-and-storage
Linde. (Agosto de 2024). Adsorption-Based Carbon Capture and CO2 Recovery. Obtenido de https://www.linde-engineering.com/products-and-services/process-plants/adsorption-and-membrane-plants/adsorption-based-carbon-capture-and-co2-recovery
Mertens, J. B.-M. (2023). Carbon capture and utilization: More than hiding CO2 for some time. Joule.
Raganati, F. M. (2021). Adsorption of Carbon Dioxide for Post-combustion Capture: A Review. Energy & Fuels, 12.845 – 12.868.
SLB. (2023). Apura Gas Separation Membrane. Obtenido de https://www.slb.com/well-production/processing-and-separation/gas-treatment/apura-gas-separation-membrane
Vattenfall. (2008). Carbon Capture and Storage- Technology, costs and way forward.
Wang, X. &. (2020). Carbon Capture From Flue Gas and the Atmosphere: A Perspective. Frontiers in Energy Research.